Table of Contents
- Executive Summary: Key Insights & 2025 Outlook
- Market Size Forecasts: 2025–2030 Growth Projections
- Technology Landscape: Current Innovations & Emerging Platforms
- Major Players & Strategic Initiatives (Company Websites Only)
- Regulatory & Standards Update: Global and Regional Perspectives
- Clinical Applications: Use Cases in Gastroenterology and Beyond
- Competitive Analysis: Differentiators and Barriers to Entry
- Investment Trends and Funding Activity
- Challenges and Risks: Technical, Regulatory, and Market Adoption
- Future Outlook: Opportunities and Game-Changers to 2030
- Sources & References
Executive Summary: Key Insights & 2025 Outlook
Endoscopic odor detection systems are poised for significant evolution in 2025, reflecting both rapid technological advances and growing clinical interest in non-visual diagnostic cues. These systems, integrating sensor arrays—often dubbed “electronic noses” or e-noses—into endoscopes, allow for the detection and analysis of volatile organic compounds (VOCs) emanating from tissues. Recent years have witnessed accelerated R&D and early-stage deployments, with 2025 expected to be a pivotal year in the transition from research prototypes to commercial clinical tools.
Several major medical device manufacturers and sensor technology firms are driving this progress. For example, Olympus Corporation and Fujifilm Holdings Corporation, both global leaders in endoscopic systems, have signaled ongoing interest in expanding their platforms to include advanced diagnostic modules, including odor detection. Meanwhile, sensor specialists such as Figaro Engineering Inc. and Sensirion AG are supplying miniaturized gas sensors compatible with medical endoscope integration.
Key 2025 trends include the integration of machine learning algorithms for real-time pattern recognition, enabling more precise differentiation between healthy and pathological tissue signatures based on VOC profiles. Clinical pilot studies in Europe and Asia are increasingly demonstrating the feasibility of these systems for early detection of gastrointestinal cancers and infections, where traditional imaging may be less conclusive. As regulatory pathways clarify, especially in the EU and Japan, early commercial launches are anticipated—targeting high-risk screening and intraoperative guidance.
The global demand for improved, non-invasive diagnostic accuracy is accelerating partnerships between hospitals, research institutes, and industry. Notably, collaborations with hospital networks are providing the large datasets necessary to train odor-detection AI, while device makers are refining sensor robustness and biocompatibility. Investment in this sector is rising, with several manufacturers expanding their production capabilities to meet anticipated demand.
Looking forward, the outlook for 2025 and the subsequent few years is strongly positive. Adoption will likely begin in specialized gastroenterology and oncology centers, with broader deployment dependent on reimbursement decisions and further clinical validation. As performance data mounts, endoscopic odor detection systems are expected to become a standard adjunct to visual diagnostics, contributing to earlier disease intervention and personalized medicine approaches.
Market Size Forecasts: 2025–2030 Growth Projections
The market for endoscopic odor detection systems is poised for significant development during the 2025–2030 period, driven by continued advances in sensor miniaturization, artificial intelligence integration, and increasing demand for enhanced diagnostic capabilities in healthcare. As of 2025, adoption remains in its early stages, with only a select group of medical device manufacturers and research-driven organizations introducing commercial or near-market solutions. However, growing clinical interest—especially in early cancer detection, infection identification, and intraoperative guidance—is expected to propel rapid market expansion.
Key players actively engaged in this field include Olympus Corporation and Fujifilm Holdings, both of which have a global presence in endoscopic technologies and have announced ongoing research collaborations targeting chemical and gas sensing integration into endoscopic platforms. These companies, along with emerging startups and university spin-offs, are developing prototypes leveraging electronic nose (e-nose) technologies and volatile organic compound (VOC) sensor arrays, designed to detect disease-specific odor signatures during endoscopic procedures.
From a market size perspective, projections for 2025 indicate a nascent but rapidly scaling segment, with initial deployments limited to high-end research hospitals and specialized clinics in Europe, North America, and East Asia. As validation studies progress and regulatory pathways become clearer—particularly in the EU, Japan, and the US—commercial availability is expected to accelerate from 2026 onwards. By 2030, optimistic scenarios suggest multi-fold growth, with the market potentially reaching several hundred million USD in annual revenues, fueled by widespread adoption for gastrointestinal, pulmonary, and urological applications.
The outlook is further strengthened by the integration of odor detection modules into existing flexible and capsule endoscope systems, a trend championed by industry leaders such as Olympus Corporation and Fujifilm Holdings. Partnerships with sensor component specialists and AI software firms are expected to accelerate time-to-market and clinical acceptance.
In summary, the 2025–2030 period is projected to be one of accelerated innovation and commercialization for endoscopic odor detection systems, moving from experimental deployments in 2025 to broader clinical integration by the decade’s end. Close collaboration between device manufacturers, clinical researchers, and regulatory bodies will be critical to realizing the full potential of this technology in routine medical diagnostics.
Technology Landscape: Current Innovations & Emerging Platforms
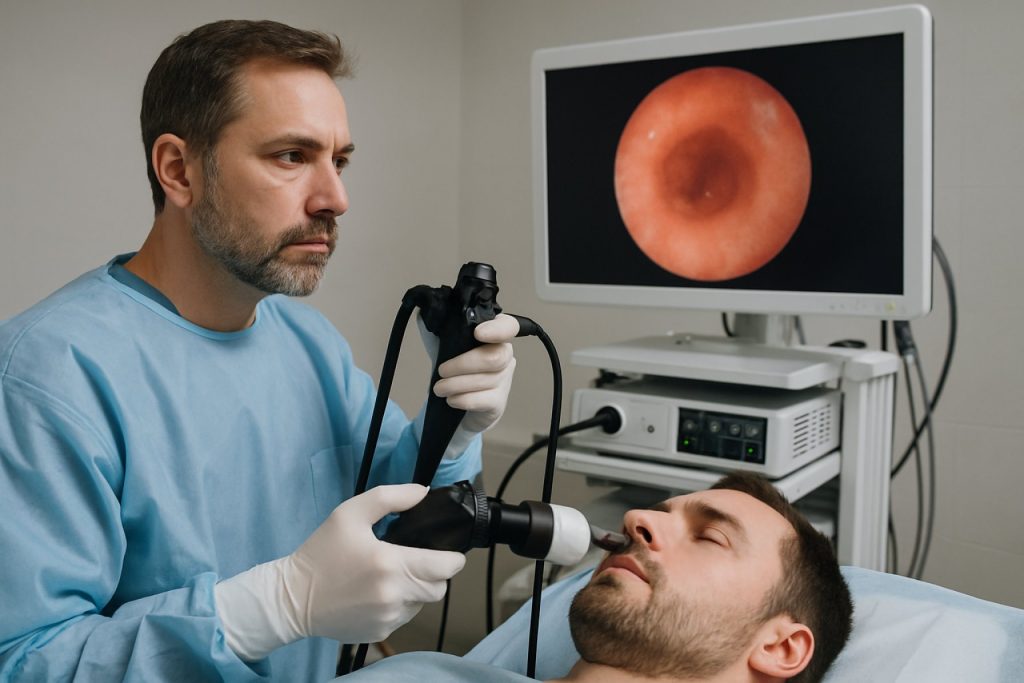
Endoscopic odor detection systems represent a rapidly advancing frontier in both medical diagnostics and industrial inspection, leveraging the integration of chemical sensors (often dubbed “electronic noses”) with flexible endoscopic platforms. These systems are designed to detect volatile organic compounds (VOCs) and other odoriferous molecules in real time, providing non-visual diagnostic cues during endoscopic procedures.
As of 2025, major strides have been reported in miniaturizing sensor arrays and improving their selectivity and sensitivity. Companies such as Olympus Corporation and Fujifilm Holdings are actively exploring the integration of advanced MEMS-based gas sensors with their endoscopy lines. These sensors are capable of detecting specific biomarkers associated with early-stage cancers, gastrointestinal disorders, and infections, thus augmenting visual diagnosis with molecular-level information.
One key area of innovation is the development of hybrid optical-chemical endoscopes, where odor sensors are embedded alongside high-definition imaging chips. These platforms can simultaneously capture tissue morphology and VOC profiles. For instance, pilot collaborations between endoscope manufacturers and sensor technology companies—such as Figaro Engineering and Sensirion AG—have yielded prototypes that can discriminate between benign and malignant lesions based on their chemical signatures.
Recent clinical studies, particularly in Europe and East Asia, have validated the feasibility of in vivo odor profiling during endoscopic procedures. Early data suggest that these systems can reduce biopsy rates and improve diagnostic confidence in cases like colorectal and gastric cancers. Regulatory engagement is also intensifying: organizations such as the International Electrotechnical Commission (IEC) are working towards standardized testing protocols for odor sensor performance within medical endoscopy platforms.
Looking ahead, the technology landscape is expected to shift towards multi-modal platforms integrating AI-powered pattern recognition with sensor data. This will enable real-time decision support during procedures, broadening the utility of endoscopic odor detection to include inflammatory and infectious diseases. Industry outlooks indicate continued investment in R&D by leading endoscope manufacturers and sensor suppliers, with commercial deployments anticipated by the late 2020s. The convergence of advances in nanomaterials, wireless data transmission, and miniaturized electronics is poised to further accelerate adoption and expand clinical as well as industrial applications.
Major Players & Strategic Initiatives (Company Websites Only)
The landscape of endoscopic odor detection systems in 2025 is characterized by a small but rapidly advancing cohort of technology leaders and medical device manufacturers. These companies are building on breakthroughs in sensor miniaturization, artificial intelligence (AI), and biocompatible materials to bring odor-sensing capabilities into minimally invasive procedures. Several strategic partnerships, clinical collaborations, and targeted R&D investments define the competitive dynamics of this sector.
A notable player is Olympus Corporation, a global leader in endoscopy equipment, which has invested in integrating advanced sensor arrays into their endoscopic platforms. Olympus has publicly highlighted its focus on enhancing diagnostic precision by leveraging multi-modal sensing, including volatile organic compound (VOC) detection within gastrointestinal (GI) endoscopy. In recent years, the company has accelerated joint research with academic partners to validate odor profiles correlated with GI pathologies, aiming for commercial deployment by the late 2020s.
Another influential entity is Fujifilm Holdings Corporation, which has a history of innovation in endoscopic imaging and surgical systems. In 2024–2025, Fujifilm announced expanded R&D programs to incorporate chemical and odor sensors alongside traditional optical tools. The company’s strategy includes collaborations with Japanese and European research hospitals to gather clinical evidence supporting the efficacy of odor detection in early cancer screening and infection monitoring.
Meanwhile, Hoya Corporation, through its subsidiary Pentax Medical, is actively developing prototypes featuring integrated electronic noses. These devices are designed to recognize specific odor signatures associated with metabolic disorders and GI infections. Hoya’s ongoing pilot studies in Asia and Europe reflect its intent to differentiate through AI-powered real-time analysis of odor data, with results expected to inform regulatory submissions by 2026.
Beyond the major incumbents, specialist sensor manufacturers such as Figaro Engineering Inc. have begun supplying miniaturized gas sensors tailored for medical endoscopy applications. Figaro collaborates with endoscope OEMs to refine sensor sensitivity and durability, key for clinical adoption.
Looking ahead, the next few years will likely see an intensification of strategic alliances between endoscope manufacturers, sensor specialists, and AI companies. This is driven by increasing demand for early, non-invasive disease detection and the growing recognition of odor signatures as valuable diagnostic biomarkers. As product pipelines mature and regulatory pathways become clearer, the sector is poised for commercial breakthroughs and broader clinical integration by the late 2020s.
Regulatory & Standards Update: Global and Regional Perspectives
The regulatory landscape for endoscopic odor detection systems is evolving as the integration of sensor-based diagnostics into minimally invasive procedures gains momentum. Traditionally, endoscopy has focused on visual diagnostics, but advances in sensor technology are enabling new capabilities, such as volatile organic compound (VOC) detection, to aid in early disease identification. In 2025, regulatory authorities in major markets are actively reviewing and updating standards to accommodate these innovations, recognizing both their diagnostic potential and unique safety considerations.
In the United States, the Food and Drug Administration (FDA) continues to classify endoscopic odor detection systems under its broader framework for medical device regulation, specifically as Class II devices when they supplement diagnostic processes. The FDA requires robust clinical evidence demonstrating that odor-detection outputs provide clinically actionable information. In recent premarket submissions, there is growing emphasis on analytical validation, biocompatibility of integrated sensors, and cybersecurity of connected systems, reflecting the increased use of electronic noses and AI-based pattern recognition in products entering the market (Olympus Corporation).
The European Union’s Medical Device Regulation (MDR), enforced since 2021, has spurred manufacturers to enhance their clinical evaluation and post-market surveillance for sensor-augmented endoscopes. In 2025, notified bodies are focusing on demonstrating the added clinical value of odor detection, as well as ensuring compliance with general safety and performance requirements, including electromagnetic compatibility and data integrity. The Fujifilm Holdings Corporation and PENTAX Medical are among the companies aligning their product development and technical documentation to meet the MDR’s enhanced scrutiny for innovative features.
- In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) is piloting new guidance for sensor-integrated endoscopes, emphasizing standards for odor sensor calibration, repeatability, and cross-contamination controls, building on the country’s established leadership in endoscopic technology (Olympus Corporation).
- In China, the National Medical Products Administration (NMPA) is updating its device classification database, with draft guidelines on the approval process for hybrid diagnostic platforms that incorporate gas analysis and AI decision support (HOYA Corporation).
Looking ahead, international harmonization efforts are underway through organizations such as the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), which are developing consensus standards for sensor performance, data security, and interoperability. As VOC-based diagnostics mature, regulatory bodies are expected to issue more detailed requirements for clinical validation, risk management, and post-market monitoring. The next few years will see closer collaboration between manufacturers and regulators to balance innovation with patient safety, shaping the global adoption of endoscopic odor detection systems.
Clinical Applications: Use Cases in Gastroenterology and Beyond
Endoscopic odor detection systems represent a breakthrough in medical diagnostics, leveraging electronic nose (e-nose) technologies to identify volatile organic compounds (VOCs) associated with disease states. In gastroenterology, these systems are increasingly integrated with endoscopic platforms to enhance real-time diagnostics. As of 2025, several clinical studies and pilot deployments are underway, especially targeting early detection of gastrointestinal (GI) cancers, inflammatory bowel disease (IBD), and Helicobacter pylori infections.
Recent events highlight the clinical momentum: in 2024, multiple centers in Europe and Asia reported on pilot studies using e-nose devices attached to endoscopes for the detection of gastric and colorectal cancer. These studies demonstrated sensitivities exceeding 85% for malignant lesion differentiation based on VOC profiles in the GI tract. For example, research collaborations with medical device manufacturers such as Olympus Corporation have enabled the integration of miniaturized sensor arrays within existing endoscopic systems, facilitating seamless workflow adoption.
Beyond neoplasia, endoscopic odor detection is being explored for its potential to identify bacterial overgrowth and metabolic disorders. Clinical use cases include non-invasive assessment of small intestinal bacterial overgrowth (SIBO) during duodenoscopy, as well as VOC-based detection of Clostridioides difficile infections. In these scenarios, companies like Fujifilm Holdings Corporation are reportedly piloting prototype systems that combine optical imaging with odor analysis, aiming to enhance diagnostic yield and reduce reliance on tissue biopsies.
Looking beyond gastroenterology, endoscopic odor detection is poised for applications in pulmonology and urology. Bronchoscopic platforms are being adapted to screen for lung cancer and chronic obstructive pulmonary disease (COPD) through breath and airway VOC analysis. Similarly, urologic endoscopes equipped with odor sensors are under evaluation for detecting bladder and prostate cancer. The modular nature of sensor arrays allows for rapid adaptation across different endoscopic specialties, as evidenced by ongoing collaborations with global medical technology suppliers such as Karl Storz SE & Co. KG.
The outlook for endoscopic odor detection systems over the next few years is marked by rapid technological refinement, miniaturization, and increased clinical validation. Industry leaders are investing in artificial intelligence algorithms to interpret complex VOC signatures, aiming for automated, point-of-care diagnostics. As regulatory approvals progress in major markets, widespread adoption is anticipated by 2027, with the promise of transforming endoscopic procedures from primarily visual assessments to comprehensive molecular diagnostics.
Competitive Analysis: Differentiators and Barriers to Entry
The competitive landscape for endoscopic odor detection systems in 2025 is shaped by several critical differentiators and significant barriers to entry. As this emerging medical technology aims to augment diagnostic precision—particularly for gastrointestinal disorders—industry players are racing to establish technological superiority and regulatory credibility.
Differentiators in this sector are anchored primarily in sensor accuracy, integration capabilities, and clinical validation. Leading manufacturers leverage proprietary sensor arrays, often based on metal-oxide semiconductor (MOS), quartz crystal microbalance (QCM), or advanced polymer technologies, to enhance volatile organic compound (VOC) detection at clinically relevant concentrations. For instance, companies such as Olympus Corporation and FUJIFILM Holdings Corporation have invested in precision multi-sensor platforms capable of discriminating between complex odor profiles, aiming to support early disease detection and characterization.
Another differentiator is the seamless integration of odor detection modules with existing endoscopic imaging systems. Firms with established endoscopy product lines have an advantage, as they can offer odor detection as an add-on to their widely adopted platforms. This is particularly evident with Olympus Corporation and FUJIFILM Holdings Corporation, both of which have publicized development efforts to integrate chemical sensing with video endoscopes, facilitating real-time, multi-modal diagnostics within familiar clinical workflows.
Clinical validation and regulatory pathways form a third crucial differentiator. Companies that can demonstrate robust clinical trial data and obtain regulatory approvals in key markets (e.g., FDA, CE mark) will gain significant first-mover advantages. Early adopters such as Hoekmine BV, which is developing biosensor-based odor detection for medical diagnostics, are actively pursuing such validation.
Barriers to entry remain substantial. First, the development of highly sensitive, selective, and stable sensors suitable for the dynamic and humid conditions inside the human body presents significant technical hurdles. Next, the regulatory landscape for novel diagnostic devices is stringent, requiring extensive preclinical and clinical demonstration of safety, efficacy, and reproducibility. Companies must also invest in intellectual property protection and navigate complex licensing environments, given the overlap with existing endoscopic and sensor patents held by large incumbents.
Additionally, new entrants face commercialization barriers—including clinician acceptance, integration into hospital procurement cycles, and reimbursement alignment. Established endoscopy brands such as Olympus Corporation and FUJIFILM Holdings Corporation benefit from deep clinical relationships and service networks, which may further limit market access for startups.
Looking ahead, the competitive field is likely to consolidate around players capable of continuous innovation, rapid clinical validation, and successful integration with mainstream endoscopic systems. Partnerships between sensor specialists and established endoscope manufacturers are expected to intensify, with the potential for new entrants to gain traction through disruptive biosensor technologies or AI-powered odor analysis platforms.
Investment Trends and Funding Activity
The investment landscape for endoscopic odor detection systems is demonstrating notable momentum as healthcare and technology sectors converge to address diagnostic limitations in minimally invasive procedures. As of 2025, venture capital and strategic corporate funding are increasingly directed toward startups and established companies developing sensor technologies and artificial intelligence (AI) integration for real-time odor analysis in endoscopy. This trend is driven by the growing evidence that volatile organic compounds (VOCs) detected during endoscopic procedures can serve as biomarkers for early-stage diseases, particularly in gastroenterology and oncology.
Several medtech companies and research institutions are spearheading the development of advanced electronic nose (e-nose) systems capable of integration with conventional and robotic endoscopes. For instance, Olympus Corporation, a leader in endoscopic imaging, has announced targeted investments in sensor miniaturization and data analytics to enhance intra-procedural diagnostics. Similarly, Intuitive Surgical, known for robotic-assisted surgery platforms, has signaled strategic interest in odor-sensing modules as part of its broader digital surgery roadmap.
Startups are also attracting early-stage funding, particularly those leveraging AI to interpret complex odor profiles. In Europe and Asia, several university spin-offs have secured seed and Series A rounds from medical device accelerators and healthtech venture funds, aiming to validate odor detection algorithms and demonstrate clinical utility in pilot hospital settings.
Governmental and intergovernmental funding is supporting translational research in this area. EU Horizon programs and national innovation agencies in countries like Japan and South Korea are providing grants to consortia working on sensor integration, device safety, and regulatory approval pathways for odor detection endoscopes. The collaboration between academic centers and industrial partners is accelerating prototype development and clinical trial readiness.
Looking ahead, the next few years are expected to see an increase in cross-sector partnerships, as sensor manufacturers, software developers, and endoscopy platform companies align to bring odor detection systems to market. Intellectual property activity is also intensifying, with a growing number of patents filed for sensor arrays, data processing techniques, and device integration methods. Mergers and acquisitions may become more frequent as established medtech firms seek to augment their diagnostic portfolios with odor detection capabilities.
With regulatory frameworks for novel diagnostic tools evolving, and early clinical results appearing promising, the influx of targeted investment and funding suggests that endoscopic odor detection systems could transition from research prototypes to commercial products and standard-of-care applications by the latter half of the decade.
Challenges and Risks: Technical, Regulatory, and Market Adoption
Endoscopic odor detection systems, which use sensor technology to identify volatile organic compounds (VOCs) and other chemical markers during endoscopic procedures, present a promising frontier in medical diagnostics. However, as the sector moves further into 2025 and beyond, several challenges and risks—technical, regulatory, and market-related—are shaping the pace and direction of adoption.
Technical Challenges remain significant. Sensor miniaturization is a major barrier: integrating highly sensitive olfactory sensors into the narrow channels of standard endoscopes requires innovations in both materials science and microfabrication. Ensuring sensor robustness and accuracy amid the humid, variable internal body environment is another hurdle, with issues such as signal drift, biofouling, and cross-reactivity with non-target compounds still being actively addressed. Companies like Olympus Corporation and Fujifilm Holdings Corporation are investing in research partnerships to enhance sensor durability and specificity while maintaining clinical usability.
Regulatory Risks are pronounced due to the novel nature of odor-based diagnostics. Devices must undergo rigorous clinical validation to meet safety and efficacy standards set by authorities such as the US FDA and the European Medicines Agency. The lack of established regulatory pathways for odor-based diagnostics can lead to delays in approvals and market entry. Additionally, harmonizing standards for sensor calibration and result interpretation across regions is a non-trivial challenge, as standards for electronic noses and similar devices are still evolving. Manufacturers like Hoya Corporation, through its Pentax Medical division, are actively engaging with regulatory bodies to develop protocols and best practices for these innovative systems.
Market Adoption Risks hinge on both clinician acceptance and cost-effectiveness. Despite promising pilot studies, widespread deployment is limited by the need for robust clinical evidence demonstrating improved diagnostic outcomes over traditional visual and histological methods. Hospitals may face high up-front equipment costs and require additional staff training, raising concerns about reimbursement and return on investment. Furthermore, integrating odor detection data into existing clinical workflows and electronic health record systems poses informatics challenges. Market leaders such as Olympus Corporation and Fujifilm Holdings Corporation are piloting early-adopter programs with major healthcare institutions to gather real-world evidence and refine business models.
Looking ahead, industry stakeholders anticipate that continued advances in sensor technology, coupled with clarity in regulatory frameworks and positive clinical trial results, will gradually address these challenges. Strategic collaborations between device manufacturers, clinical researchers, and regulatory authorities are expected to be crucial in overcoming barriers and achieving broader market penetration in the late 2020s.
Future Outlook: Opportunities and Game-Changers to 2030
The coming years to 2030 are poised to be transformative for endoscopic odor detection systems, with significant advancements expected in sensor technology, artificial intelligence (AI), and clinical integration. As minimally invasive diagnostics continue to evolve, odor detection is emerging as a promising adjunct to traditional imaging and tissue sampling. The integration of highly sensitive gas sensors—often referred to as “electronic noses”—into endoscopic platforms could revolutionize the early diagnosis of gastrointestinal and respiratory diseases by detecting volatile organic compounds (VOCs) associated with pathological changes.
Recent developments from industry leaders in sensor technology indicate an acceleration in miniaturization, sensitivity, and selectivity of chemical sensors suitable for endoscopic use. Companies such as Figaro Engineering Inc. and Sensirion AG are making strides in MEMS-based gas sensor development, which are being adapted for medical device integration. These sensors enable real-time analysis of breath and tissue-emitted VOCs during endoscopic procedures, offering clinicians additional diagnostic data without the need for invasive biopsies.
Artificial intelligence is expected to be a game-changer, enabling rapid interpretation of complex chemical signatures detected by these sensors. Collaborative initiatives are underway between sensor manufacturers and medical device companies such as Olympus Corporation and PENTAX Medical, focusing on embedding AI-driven pattern recognition into endoscopic platforms. These efforts aim to distinguish between benign and malignant VOC profiles, supporting earlier and more accurate diagnosis of cancers and infections.
From a clinical perspective, several pilot studies and early commercial implementations are anticipated by 2027, particularly in specialized centers focused on gastroenterology and pulmonology. Regulatory pathways for these systems are expected to clarify as the technology matures, with standards bodies and industry groups, such as the Association for the Advancement of Medical Instrumentation (AAMI), likely to play a key role in shaping guidelines for integration, safety, and efficacy.
Looking toward 2030, the convergence of advanced sensor technology, real-time data analytics, and AI-driven interpretation is expected to move endoscopic odor detection from experimental to routine clinical use in select indications. The potential for non-invasive, on-the-spot diagnostics could reduce the need for tissue biopsies and enhance patient outcomes, cementing odor detection as a core component of next-generation endoscopic systems.



