How CRISPR-Cas9 Epigenetic Modulation is Revolutionizing Induced Pluripotent Stem Cell (iPSC) Rejuvenation—A Deep Dive into the Science and Future Potential
- Introduction to iPSC Rejuvenation and Epigenetic Modulation
- CRISPR-Cas9: Mechanisms and Innovations in Epigenetic Editing
- Key Epigenetic Targets for iPSC Rejuvenation
- Recent Advances: Case Studies and Experimental Results
- Challenges and Limitations in CRISPR-Cas9 Epigenetic Modulation
- Therapeutic Implications and Regenerative Medicine Applications
- Ethical Considerations and Regulatory Landscape
- Future Directions: Emerging Technologies and Research Frontiers
- Conclusion: The Road Ahead for iPSC Rejuvenation via CRISPR-Cas9
- Sources & References
Introduction to iPSC Rejuvenation and Epigenetic Modulation
Induced pluripotent stem cells (iPSCs) are somatic cells reprogrammed to a pluripotent state, enabling them to differentiate into various cell types and offering immense potential for regenerative medicine and disease modeling. However, iPSCs often retain residual epigenetic memory from their cells of origin, which can limit their full rejuvenation and differentiation potential. Epigenetic modulation—altering chemical modifications on DNA or histones without changing the underlying genetic code—has emerged as a promising strategy to enhance iPSC rejuvenation by resetting these epigenetic marks to a more embryonic-like state.
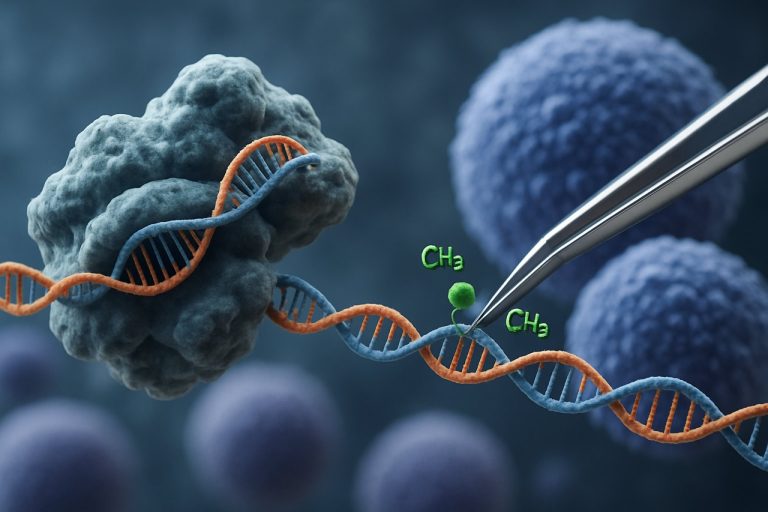
The advent of CRISPR-Cas9 technology has revolutionized the field of epigenetic editing. Unlike traditional CRISPR applications that induce double-strand breaks for gene editing, CRISPR-based epigenetic modulation employs catalytically inactive Cas9 (dCas9) fused to epigenetic effector domains. This system allows for precise, locus-specific modification of epigenetic marks, such as DNA methylation or histone acetylation, thereby enabling targeted reprogramming of gene expression profiles in iPSCs. Such targeted interventions can erase age-associated or lineage-specific epigenetic signatures, promoting a more complete rejuvenation of iPSCs and improving their functional properties.
Recent studies have demonstrated the feasibility of using CRISPR-dCas9 systems to modulate key rejuvenation-associated genes and epigenetic regulators in iPSCs, paving the way for more efficient and safer stem cell therapies. As the field advances, the integration of CRISPR-Cas9 epigenetic modulation with iPSC technology holds significant promise for overcoming current limitations in cellular reprogramming and for the development of personalized regenerative medicine approaches (Nature Reviews Genetics, Cell Stem Cell).
CRISPR-Cas9: Mechanisms and Innovations in Epigenetic Editing
The CRISPR-Cas9 system, originally developed for precise genome editing, has been innovatively adapted for targeted epigenetic modulation, offering new avenues for the rejuvenation of induced pluripotent stem cells (iPSCs). Unlike traditional CRISPR-Cas9, which introduces double-strand breaks to edit DNA sequences, epigenetic editing employs a catalytically inactive Cas9 (dCas9) fused to epigenetic effector domains. This allows for locus-specific modification of chromatin states—such as DNA methylation or histone acetylation—without altering the underlying genetic code. Such precision is crucial for iPSC rejuvenation, where resetting the epigenetic landscape can restore youthful gene expression profiles and enhance cellular function.
Recent innovations include dCas9 fused to DNA methyltransferases or demethylases, as well as histone acetyltransferases or deacetylases, enabling the reversible activation or repression of target genes implicated in aging and senescence. For example, targeted demethylation of promoters for pluripotency genes can reactivate their expression, improving iPSC quality and differentiation potential. Additionally, multiplexed epigenetic editing—simultaneously targeting multiple loci—has been demonstrated, further enhancing the rejuvenation process by orchestrating complex gene regulatory networks Nature Reviews Genetics.
These advances are complemented by improved delivery systems, such as ribonucleoprotein complexes and viral vectors, which increase editing efficiency and reduce off-target effects. Collectively, CRISPR-Cas9-based epigenetic editing represents a transformative approach for iPSC rejuvenation, with the potential to overcome limitations of conventional reprogramming and pave the way for regenerative therapies Cell Stem Cell.
Key Epigenetic Targets for iPSC Rejuvenation
A critical aspect of leveraging CRISPR-Cas9 for epigenetic modulation in induced pluripotent stem cell (iPSC) rejuvenation is the identification and targeting of key epigenetic regulators that govern cellular aging and pluripotency. Among the most prominent targets are DNA methylation patterns, histone modifications, and non-coding RNA loci, all of which play pivotal roles in maintaining or erasing cellular memory during reprogramming. For instance, the methylation status of the p16INK4a promoter, a well-known senescence marker, has been shown to influence the proliferative capacity and rejuvenation potential of iPSCs. CRISPR-dCas9 systems fused with DNA demethylases or methyltransferases can be precisely directed to such loci to modulate their epigenetic state, thereby enhancing reprogramming efficiency and cellular youthfulness Nature Reviews Genetics.
Another key target is the regulation of histone modifications, such as H3K9me3 and H3K27me3, which are associated with heterochromatin formation and gene silencing. Targeted editing of these marks at specific genomic regions using CRISPR-dCas9 fused to histone-modifying enzymes can reactivate pluripotency genes or repress aging-associated genes, facilitating a more robust rejuvenation process Cell Stem Cell. Additionally, long non-coding RNAs (lncRNAs) that modulate chromatin architecture and gene expression are emerging as promising CRISPR targets for fine-tuning the epigenetic landscape of iPSCs Nature Cell Biology.
Overall, the strategic selection and precise modulation of these epigenetic targets using CRISPR-Cas9-based tools hold significant promise for advancing iPSC rejuvenation and the development of regenerative therapies.
Recent Advances: Case Studies and Experimental Results
Recent years have witnessed significant progress in leveraging CRISPR-Cas9-based epigenetic modulation to rejuvenate induced pluripotent stem cells (iPSCs). Notably, several studies have demonstrated the feasibility of using catalytically inactive Cas9 (dCas9) fused to epigenetic modifiers to target and remodel specific chromatin regions, thereby reversing age-associated epigenetic marks in iPSCs. For example, a landmark study by Nature reported the use of dCas9-TET1 to demethylate the promoter regions of key pluripotency genes, resulting in enhanced reprogramming efficiency and improved cellular rejuvenation markers.
Another pivotal experiment published by Cell Stem Cell utilized dCas9-p300 to acetylate histone H3K27 at loci associated with youthful gene expression profiles. This targeted epigenetic editing not only restored youthful transcriptional signatures but also improved mitochondrial function and reduced senescence-associated phenotypes in iPSCs derived from aged donors.
Furthermore, a recent preclinical study by Science demonstrated that multiplexed CRISPR-dCas9 epigenetic editing could simultaneously modulate multiple aging-related pathways, leading to synergistic effects on iPSC rejuvenation. These findings collectively underscore the potential of CRISPR-Cas9 epigenetic modulation as a precise and versatile tool for reversing cellular aging in iPSCs, paving the way for future translational applications in regenerative medicine and age-related disease modeling.
Challenges and Limitations in CRISPR-Cas9 Epigenetic Modulation
Despite the transformative potential of CRISPR-Cas9-based epigenetic modulation in rejuvenating induced pluripotent stem cells (iPSCs), several challenges and limitations persist. One major concern is off-target effects, where the CRISPR-Cas9 system may inadvertently bind to and modify unintended genomic loci, potentially leading to unpredictable epigenetic changes and genomic instability. This is particularly critical in the context of iPSC rejuvenation, as even minor off-target modifications can compromise pluripotency or trigger oncogenic pathways Nature Reviews Genetics.
Another limitation lies in the efficiency and specificity of epigenetic editing. While catalytically dead Cas9 (dCas9) fused to epigenetic modifiers can target specific loci, the degree and persistence of epigenetic changes—such as DNA methylation or histone modification—are often variable and may not fully recapitulate the youthful epigenetic landscape required for robust iPSC rejuvenation Cell Stem Cell. Additionally, the delivery of CRISPR-dCas9 components into iPSCs remains technically challenging, with viral vectors posing risks of insertional mutagenesis and non-viral methods often suffering from low efficiency.
Immunogenicity is another concern, as the introduction of exogenous proteins such as Cas9 can elicit immune responses, particularly in clinical applications. Furthermore, the long-term stability and safety of epigenetically modified iPSCs have yet to be fully established, raising questions about their suitability for therapeutic use U.S. Food & Drug Administration. Addressing these challenges is essential for the safe and effective translation of CRISPR-Cas9 epigenetic modulation strategies in iPSC rejuvenation.
Therapeutic Implications and Regenerative Medicine Applications
The application of CRISPR-Cas9-based epigenetic modulation in induced pluripotent stem cell (iPSC) rejuvenation holds significant promise for therapeutic interventions and regenerative medicine. By precisely targeting and modifying epigenetic marks—such as DNA methylation and histone modifications—at specific genomic loci, researchers can reverse age-associated epigenetic changes in iPSCs, thereby enhancing their pluripotency, genomic stability, and differentiation potential. This rejuvenation process is critical for generating high-quality iPSCs suitable for cell replacement therapies, disease modeling, and drug screening.
In regenerative medicine, rejuvenated iPSCs can be differentiated into various cell types with improved functionality and reduced risk of senescence or tumorigenicity, addressing major safety concerns in clinical applications. For example, epigenetic editing of genes involved in cellular aging, such as p16INK4a or TERT, can restore youthful gene expression profiles and telomere length, enhancing the therapeutic efficacy of derived cells. Moreover, CRISPR-dCas9 fusion proteins tethered to epigenetic modifiers enable reversible and locus-specific modulation, minimizing off-target effects and permanent genomic alterations, which is crucial for clinical translation Nature Reviews Genetics.
The ability to rejuvenate patient-derived iPSCs also opens avenues for personalized regenerative therapies, where autologous cells can be rejuvenated, corrected for disease-causing mutations, and differentiated into functional tissues for transplantation. This approach has the potential to treat a wide range of degenerative diseases, including neurodegenerative disorders, cardiovascular diseases, and diabetes, by providing a renewable source of youthful, patient-specific cells Cell Stem Cell. As the technology matures, integrating CRISPR-Cas9 epigenetic modulation with iPSC-based therapies could revolutionize the landscape of regenerative medicine.
Ethical Considerations and Regulatory Landscape
The application of CRISPR-Cas9-mediated epigenetic modulation in induced pluripotent stem cell (iPSC) rejuvenation presents significant ethical and regulatory challenges. Unlike traditional gene editing, epigenetic modulation does not alter the DNA sequence but instead modifies gene expression through reversible changes, such as DNA methylation or histone modification. While this may reduce some concerns associated with permanent genetic alterations, the long-term effects and potential for off-target impacts remain uncertain, raising questions about safety and unintended consequences in clinical applications.
Ethically, the use of CRISPR-Cas9 in iPSC rejuvenation intersects with debates on human enhancement, consent, and the potential for germline transmission if rejuvenated cells are used in reproductive contexts. There is also concern about equitable access to such advanced therapies, which could exacerbate existing health disparities. The possibility of creating “designer” cells or tissues further complicates the ethical landscape, necessitating robust oversight and public engagement.
Regulatory frameworks for CRISPR-based epigenetic interventions are still evolving. In the United States, the U.S. Food and Drug Administration oversees gene therapy and cell-based products, but specific guidelines for epigenetic editing are under development. The European Medicines Agency similarly regulates advanced therapy medicinal products, including those involving genome and epigenome editing. Internationally, organizations such as the World Health Organization have called for global standards and governance to address the unique risks and ethical dilemmas posed by these technologies.
As research progresses, ongoing dialogue among scientists, ethicists, regulators, and the public will be essential to ensure responsible innovation and the safe, equitable deployment of CRISPR-Cas9 epigenetic modulation in iPSC rejuvenation.
Future Directions: Emerging Technologies and Research Frontiers
The future of CRISPR-Cas9 epigenetic modulation in induced pluripotent stem cell (iPSC) rejuvenation is poised for significant advancements, driven by emerging technologies and novel research frontiers. One promising direction is the integration of CRISPR-based epigenetic editing with single-cell multi-omics, enabling precise mapping and manipulation of chromatin states, DNA methylation, and histone modifications at the individual cell level. This approach could unravel the heterogeneity of iPSC populations and optimize rejuvenation protocols for enhanced cellular function and longevity Nature Reviews Genetics.
Another frontier involves the development of next-generation CRISPR systems, such as base editors and prime editors, which allow for more refined and reversible epigenetic modifications without introducing double-strand breaks. These tools may minimize off-target effects and genomic instability, addressing key safety concerns in clinical applications Cell Stem Cell. Additionally, the use of programmable epigenetic effectors, such as dCas9 fused to chromatin-modifying enzymes, is being explored to reset age-associated epigenetic marks and restore youthful gene expression profiles in iPSCs Science.
Looking ahead, the convergence of artificial intelligence and machine learning with CRISPR technologies is expected to accelerate the identification of rejuvenation targets and predict optimal editing strategies. Furthermore, advances in delivery systems, such as nanoparticle-based or virus-free methods, will enhance the efficiency and safety of epigenetic editing in iPSCs. Collectively, these innovations hold the potential to revolutionize regenerative medicine and age-related disease modeling by enabling precise, safe, and durable iPSC rejuvenation Nature Biotechnology.
Conclusion: The Road Ahead for iPSC Rejuvenation via CRISPR-Cas9
The application of CRISPR-Cas9-mediated epigenetic modulation in the rejuvenation of induced pluripotent stem cells (iPSCs) represents a transformative frontier in regenerative medicine. By enabling precise, locus-specific editing of epigenetic marks, this technology offers the potential to reset age-associated molecular signatures, enhance iPSC quality, and improve their therapeutic utility. Recent advances have demonstrated the feasibility of targeting key epigenetic regulators to restore youthful gene expression profiles and functional capacities in iPSCs, thereby addressing limitations such as residual epigenetic memory and incomplete reprogramming Nature Reviews Genetics.
Looking ahead, several challenges and opportunities define the road to clinical translation. Ensuring the specificity and safety of CRISPR-based epigenetic editing remains paramount, as off-target effects and unintended chromatin alterations could compromise cell function or safety U.S. Food & Drug Administration. The development of next-generation CRISPR tools with improved fidelity, as well as robust delivery systems, will be critical for advancing this field. Furthermore, integrating multi-omics approaches to monitor and validate rejuvenation outcomes will enhance our understanding of the underlying mechanisms and facilitate regulatory approval National Human Genome Research Institute.
Ultimately, the convergence of CRISPR-Cas9 technology and iPSC biology holds immense promise for personalized cell therapies, disease modeling, and the study of human aging. Continued interdisciplinary collaboration and ethical oversight will be essential to realize the full therapeutic potential of iPSC rejuvenation via epigenetic modulation.