Hereditary Angioedema Explained: Understanding the Genetics, Symptoms, and Breakthrough Treatments of This Rare but Life-Threatening Condition (2025)
- Overview of Hereditary Angioedema (HAE): Definition and Epidemiology
- Genetic Causes and Inheritance Patterns of HAE
- Clinical Manifestations: Recognizing the Signs and Symptoms
- Diagnostic Criteria and Advances in Testing
- Current Treatment Options and Emergency Management
- Emerging Therapies and Research Pipelines
- Patient Quality of Life and Psychosocial Impact
- Global Awareness, Advocacy, and Support Organizations
- Market Trends and Forecast: Growth in HAE Therapeutics (Estimated 8-12% CAGR through 2030)
- Future Outlook: Innovations, Unmet Needs, and Public Health Implications
- Sources & References
Overview of Hereditary Angioedema (HAE): Definition and Epidemiology
Hereditary Angioedema (HAE) is a rare, potentially life-threatening genetic disorder characterized by recurrent episodes of severe swelling (angioedema) in various parts of the body, including the extremities, face, gastrointestinal tract, and airway. The condition is most commonly caused by a deficiency or dysfunction of the C1 esterase inhibitor (C1-INH), a protein that regulates several pathways involved in inflammation and vascular permeability. HAE is typically inherited in an autosomal dominant manner, meaning a single copy of the altered gene in each cell is sufficient to cause the disorder. There are three recognized types: Type I (C1-INH deficiency), Type II (C1-INH dysfunction), and Type III (normal C1-INH, often associated with mutations in the F12 gene or other factors).
Epidemiologically, HAE is considered an ultra-rare disease, with an estimated prevalence ranging from 1 in 30,000 to 1 in 50,000 individuals worldwide. Recent data from patient registries and national health authorities suggest that the true prevalence may be underestimated due to misdiagnosis or underdiagnosis, as symptoms can mimic more common conditions such as allergic reactions or gastrointestinal disorders. Both males and females are affected, though some studies indicate that women may experience more frequent or severe attacks, possibly due to hormonal influences.
In 2025, the global HAE community continues to benefit from improved awareness, earlier diagnosis, and expanded access to effective therapies. Organizations such as the Hereditary Angioedema International (HAEi), a leading global patient advocacy group, and the American College of Allergy, Asthma & Immunology (ACAAI), play pivotal roles in education, research, and support for patients and healthcare professionals. National and international HAE registries, supported by these organizations and others, are providing increasingly robust epidemiological data, helping to refine prevalence estimates and inform public health strategies.
Looking ahead, ongoing efforts in 2025 and beyond are focused on further improving diagnostic accuracy, particularly through the use of genetic testing and biomarker identification. These advances are expected to reduce diagnostic delays, which historically have averaged 8–10 years from symptom onset to diagnosis. Additionally, the continued expansion of newborn screening programs and family cascade testing may lead to earlier identification of affected individuals, ultimately improving outcomes and quality of life for people living with HAE.
Genetic Causes and Inheritance Patterns of HAE
Hereditary Angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of severe swelling in various body tissues, most commonly affecting the skin, gastrointestinal tract, and airways. The genetic basis of HAE has been well established, with the majority of cases resulting from mutations in the SERPING1 gene, which encodes the C1 esterase inhibitor (C1-INH) protein. Deficiency or dysfunction of C1-INH leads to uncontrolled activation of the complement and contact systems, resulting in increased bradykinin production and subsequent vascular permeability.
As of 2025, research continues to refine the understanding of HAE’s genetic underpinnings. Approximately 85% of HAE cases (Type I and Type II) are linked to autosomal dominant mutations in SERPING1. Type I HAE is characterized by low levels of functional C1-INH, while Type II involves normal or elevated levels of dysfunctional C1-INH. Both types follow an autosomal dominant inheritance pattern, meaning a single mutated allele is sufficient to cause the disorder, and each child of an affected parent has a 50% chance of inheriting the mutation. However, de novo mutations—those arising spontaneously—account for up to 25% of new HAE cases, underscoring the importance of genetic testing even in the absence of family history.
A third, less common form, HAE with normal C1-INH (formerly known as Type III), is associated with mutations in other genes, such as F12 (encoding coagulation factor XII), PLG (plasminogen), ANGPT1 (angiopoietin-1), and KNG1 (kininogen-1). These variants also exhibit autosomal dominant inheritance but may display variable penetrance and expressivity, complicating diagnosis and genetic counseling. Ongoing studies in 2025 are investigating additional genetic modifiers and epigenetic factors that may influence disease severity and response to therapy.
- The National Center for Biotechnology Information (NCBI) maintains updated databases cataloging known pathogenic variants in HAE-related genes, supporting clinicians and researchers in variant interpretation.
- The US Hereditary Angioedema Association (HAEA), a leading patient advocacy and research organization, provides educational resources and supports ongoing genetic research and patient registries.
- The World Health Organization (WHO) recognizes the importance of rare disease genomics, including HAE, in its global health strategies.
Looking ahead, advances in next-generation sequencing and international collaboration are expected to further elucidate the genetic landscape of HAE. This will likely improve diagnostic accuracy, enable earlier intervention, and inform the development of targeted therapies tailored to specific genetic subtypes over the next several years.
Clinical Manifestations: Recognizing the Signs and Symptoms
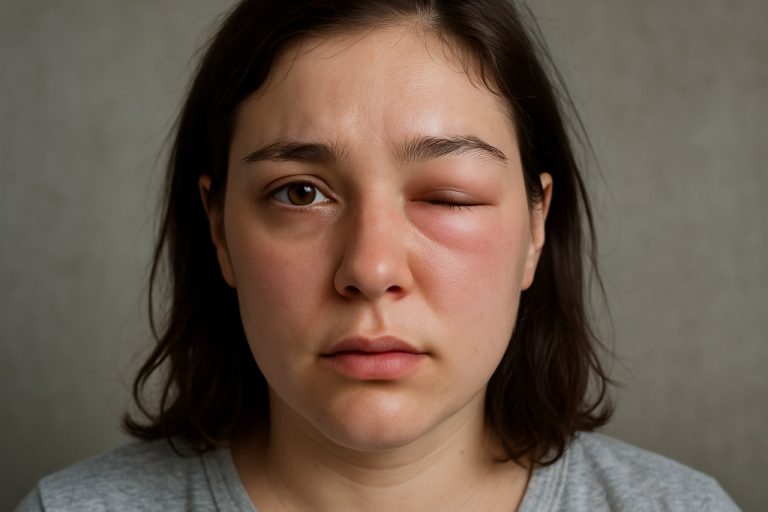
Hereditary angioedema (HAE) is a rare, potentially life-threatening genetic disorder characterized by recurrent episodes of severe swelling (angioedema) in various parts of the body, including the skin, gastrointestinal tract, and upper airways. As of 2025, clinical recognition of HAE remains a critical challenge due to its variable presentation and overlap with more common conditions such as allergic angioedema. Early and accurate identification of HAE is essential to prevent morbidity and mortality, particularly from laryngeal attacks, which can cause fatal airway obstruction.
The classic clinical manifestations of HAE include non-pitting, non-pruritic swelling of the extremities, face, genitals, and trunk. Abdominal attacks, which occur in up to 90% of patients, present with severe pain, vomiting, and sometimes ascites, often leading to unnecessary surgical interventions before diagnosis. Laryngeal edema, though less frequent, is the most feared complication due to its rapid progression and risk of asphyxiation. Unlike histaminergic angioedema, HAE attacks are not associated with urticaria or response to antihistamines or corticosteroids, which is a key diagnostic clue.
Recent data highlight that the average delay from symptom onset to diagnosis remains significant, often exceeding 8 years globally, though increased awareness and education efforts are beginning to shorten this interval in some regions. The American College of Allergy, Asthma & Immunology and the World Allergy Organization have emphasized the importance of recognizing prodromal symptoms—such as erythema marginatum (a serpiginous rash), tingling, or fatigue—that may precede attacks by several hours. These early signs, if identified, can prompt timely intervention and reduce attack severity.
- Cutaneous swelling: Most common, often affecting hands, feet, face, and genitals.
- Abdominal pain: Severe, colicky pain with possible nausea, vomiting, and diarrhea.
- Laryngeal edema: Hoarseness, throat tightness, and risk of airway compromise.
- Prodromal symptoms: Fatigue, rash (erythema marginatum), or tingling sensations.
Looking ahead, ongoing educational initiatives and the development of rapid diagnostic assays are expected to further improve early recognition of HAE in the next few years. The US Hereditary Angioedema Association and similar organizations worldwide are actively promoting awareness campaigns and clinician training. These efforts, combined with advances in telemedicine and digital health tools, are anticipated to reduce diagnostic delays and improve patient outcomes by 2025 and beyond.
Diagnostic Criteria and Advances in Testing
Hereditary angioedema (HAE) is a rare, potentially life-threatening genetic disorder characterized by recurrent episodes of severe swelling in various body tissues. Accurate and timely diagnosis is critical, as misdiagnosis can lead to inappropriate treatment and increased morbidity. As of 2025, diagnostic criteria for HAE continue to be refined, with a focus on integrating clinical presentation, family history, and advanced laboratory testing.
The cornerstone of HAE diagnosis remains the measurement of complement component 4 (C4) and C1 inhibitor (C1-INH) levels and function. Most patients with HAE types I and II exhibit low C4 and either low C1-INH levels (type I) or dysfunctional C1-INH (type II). In recent years, the identification of HAE with normal C1-INH (formerly type III) has prompted the inclusion of genetic testing for mutations in genes such as F12, PLG, and ANGPT1 in diagnostic algorithms. The American Academy of Allergy, Asthma & Immunology and the World Allergy Organization both recommend a stepwise approach, starting with clinical suspicion and family history, followed by laboratory and, if indicated, genetic testing.
Recent advances in testing are improving both the speed and accuracy of HAE diagnosis. High-sensitivity assays for C1-INH function and next-generation sequencing (NGS) panels are increasingly available in specialized centers, allowing for the detection of rare and novel mutations. In 2024 and 2025, several academic and clinical laboratories have begun to offer rapid NGS-based panels that can identify known and emerging HAE-associated mutations within days, rather than weeks. This is particularly important for patients with atypical presentations or those with normal C1-INH levels, where traditional assays may be inconclusive.
Point-of-care testing is also under investigation, with pilot studies evaluating the feasibility of rapid C4 and C1-INH assays in emergency and outpatient settings. These developments could significantly reduce diagnostic delays, which currently average several years from symptom onset. The US Hereditary Angioedema Association, a leading patient advocacy and research organization, is actively supporting initiatives to increase awareness and access to advanced diagnostic tools.
Looking ahead, the integration of artificial intelligence (AI) and machine learning into diagnostic workflows is anticipated to further enhance early recognition of HAE. Algorithms trained on large datasets may help identify subtle clinical patterns and recommend targeted testing, especially in primary care. As these technologies mature and become more widely adopted, the outlook for timely and accurate HAE diagnosis in 2025 and beyond is increasingly optimistic.
Current Treatment Options and Emergency Management
Hereditary angioedema (HAE) is a rare genetic disorder characterized by recurrent episodes of severe swelling in various body parts, including the extremities, face, gastrointestinal tract, and airway. As of 2025, the management of HAE has advanced significantly, with a focus on both acute attack treatment and long-term prophylaxis. The primary goal remains rapid symptom control and prevention of life-threatening laryngeal attacks.
Current treatment options for acute HAE attacks include plasma-derived and recombinant C1 esterase inhibitor (C1-INH) concentrates, bradykinin B2 receptor antagonists, and kallikrein inhibitors. C1-INH replacement therapies, such as those produced by Takeda and CSL, remain the cornerstone for on-demand management. These therapies can be administered intravenously or subcutaneously, allowing for both hospital-based and self-administration at home. Icatibant, a bradykinin B2 receptor antagonist, is widely used for its rapid subcutaneous administration and efficacy in resolving symptoms, while ecallantide, a plasma kallikrein inhibitor, is another option for acute attacks.
For long-term prophylaxis, several options are available in 2025. Subcutaneous C1-INH products and lanadelumab, a monoclonal antibody targeting plasma kallikrein, have demonstrated significant reductions in attack frequency and improved quality of life for patients. Oral therapies, such as berotralstat, have also gained traction, offering a convenient daily regimen for patients who prefer non-injectable options. These advances have been supported by ongoing clinical trials and real-world data, which continue to inform best practices and refine treatment algorithms.
Emergency management protocols emphasize the importance of early recognition and prompt treatment of attacks, particularly those involving the airway. Patients are advised to carry emergency medication and have an individualized action plan. Healthcare providers are increasingly trained to recognize HAE symptoms and initiate appropriate therapy without delay. The World Allergy Organization and American Academy of Allergy, Asthma & Immunology provide updated guidelines and educational resources to support clinicians and patients.
Looking ahead, the outlook for HAE management is promising. Ongoing research is focused on developing novel therapies with improved efficacy, safety, and convenience, including gene therapies and next-generation biologics. The continued collaboration between patient advocacy groups, pharmaceutical companies, and regulatory agencies is expected to further enhance access to effective treatments and improve patient outcomes in the coming years.
Emerging Therapies and Research Pipelines
Hereditary angioedema (HAE) is a rare, potentially life-threatening genetic disorder characterized by recurrent episodes of severe swelling in various body parts. The therapeutic landscape for HAE has evolved rapidly in recent years, and 2025 marks a period of significant innovation, with several emerging therapies and research pipelines aiming to address unmet needs in both acute and prophylactic treatment.
Current standard-of-care therapies include C1 esterase inhibitor (C1-INH) replacement, kallikrein inhibitors, and bradykinin receptor antagonists. However, limitations such as frequent dosing, intravenous or subcutaneous administration, and breakthrough attacks persist. In response, the research community and biopharmaceutical industry are advancing novel approaches to improve efficacy, safety, and patient convenience.
- Oral Therapies: The development of oral kallikrein inhibitors represents a major advance. Berotralstat, the first once-daily oral prophylactic approved in several regions, has demonstrated sustained efficacy and safety. Ongoing research is focused on next-generation oral agents with improved pharmacokinetics and tolerability, aiming to further reduce attack frequency and enhance quality of life for patients.
- Gene Therapy: Gene therapy is a promising frontier for HAE, with preclinical and early-phase clinical trials underway. These approaches seek to provide a long-term or potentially curative solution by restoring functional C1-INH expression. Several academic centers and biotechnology companies are collaborating to optimize vector delivery and minimize immunogenicity, with initial human data anticipated within the next few years.
- Monoclonal Antibodies: Monoclonal antibodies targeting plasma kallikrein or other mediators of the bradykinin pathway are in various stages of clinical development. These agents offer the potential for infrequent dosing (monthly or longer intervals) and subcutaneous administration, which could significantly improve adherence and patient satisfaction.
- Pediatric and Special Populations: Research is increasingly focused on expanding indications for existing and novel therapies to pediatric patients and those with comorbidities, addressing a critical gap in care.
The outlook for HAE treatment in 2025 and beyond is optimistic, with a robust pipeline of innovative therapies poised to transform disease management. Regulatory agencies such as the U.S. Food and Drug Administration and the European Medicines Agency are actively engaging with stakeholders to expedite the review of breakthrough therapies. Patient advocacy organizations, including the U.S. Hereditary Angioedema Association, play a pivotal role in shaping research priorities and ensuring patient-centric development. As these emerging therapies progress through clinical trials, the HAE community anticipates improved outcomes, greater convenience, and the possibility of long-term remission or cure.
Patient Quality of Life and Psychosocial Impact
Hereditary Angioedema (HAE) is a rare, potentially life-threatening genetic disorder characterized by recurrent episodes of severe swelling in various body parts, including the extremities, face, gastrointestinal tract, and airway. The unpredictable and often debilitating nature of HAE attacks has a profound impact on patient quality of life and psychosocial well-being, a focus that has gained increasing attention in 2025 and is expected to remain a priority in the coming years.
Recent patient surveys and clinical studies highlight that individuals with HAE continue to experience significant burdens despite advances in prophylactic and on-demand therapies. Many patients report persistent anxiety related to the unpredictability of attacks, which can disrupt daily activities, employment, education, and social relationships. The fear of laryngeal attacks, which can be fatal without prompt treatment, remains a major source of psychological distress. According to data from the Hereditary Angioedema International (HAEi), a global patient organization, over 60% of patients surveyed in 2024 indicated that HAE negatively affects their mental health, with common issues including depression, social isolation, and reduced self-esteem.
The impact on quality of life is further compounded by the need for ongoing disease management, including regular medication, emergency preparedness, and frequent healthcare interactions. The American College of Allergy, Asthma & Immunology (ACAAI) emphasizes that even with newer therapies, many patients experience treatment fatigue and concerns about long-term side effects, which can contribute to emotional burden and reduced adherence.
In response, patient advocacy groups and clinical organizations are intensifying efforts to address the psychosocial aspects of HAE. Initiatives in 2025 include expanded access to mental health resources, peer support networks, and educational programs designed to empower patients and caregivers. The US Hereditary Angioedema Association (HAEA) has launched new digital platforms and counseling services to facilitate community engagement and provide psychological support.
Looking ahead, the integration of patient-reported outcome measures (PROMs) into clinical practice and research is expected to enhance understanding of the real-world impact of HAE and guide the development of more holistic care models. Regulatory agencies such as the U.S. Food and Drug Administration (FDA) are encouraging the inclusion of quality-of-life endpoints in clinical trials, reflecting a broader shift toward patient-centered care. As novel therapies continue to emerge, ongoing collaboration between healthcare providers, researchers, and patient organizations will be essential to improving both the physical and psychosocial outcomes for individuals living with HAE.
Global Awareness, Advocacy, and Support Organizations
Global awareness and advocacy for Hereditary Angioedema (HAE) have seen significant progress in recent years, with 2025 marking a period of increased international collaboration and patient empowerment. HAE, a rare genetic disorder characterized by recurrent episodes of severe swelling, has historically suffered from underdiagnosis and limited public understanding. However, the concerted efforts of patient organizations, medical societies, and global health authorities are reshaping the landscape.
At the forefront is the HAE International (HAEi), a non-profit global umbrella organization dedicated to supporting HAE patients and their families. HAEi coordinates awareness campaigns, educational initiatives, and advocacy efforts across more than 100 member countries. In 2025, HAEi continues to expand its reach, focusing on underserved regions and promoting the annual HAE Day (May 16), which unites the global community in raising awareness and sharing patient stories.
National organizations play a pivotal role in local advocacy and support. For example, the US Hereditary Angioedema Association (HAEA) provides resources, patient education, and access to clinical trials in the United States. Similarly, the HAE UK and Angioedema Association of Germany (AEED) are instrumental in Europe, working closely with healthcare providers to improve diagnosis rates and ensure access to modern therapies.
In 2025, these organizations are leveraging digital platforms to enhance outreach. Virtual support groups, telemedicine consultations, and online educational materials have become standard, reducing barriers for patients in remote or resource-limited settings. HAEi’s Global Access Program, launched in previous years, continues to facilitate access to life-saving medications in low- and middle-income countries, addressing disparities in treatment availability.
Collaboration with scientific and medical bodies is also intensifying. The World Allergy Organization (WAO) and the European Academy of Allergy and Clinical Immunology (EAACI) regularly update clinical guidelines and host international congresses, fostering knowledge exchange between clinicians, researchers, and patient advocates.
Looking ahead, the outlook for HAE awareness and advocacy is optimistic. The momentum generated by global and national organizations is expected to drive earlier diagnosis, equitable access to therapies, and improved quality of life for patients worldwide. Continued partnerships between patient groups, healthcare professionals, and regulatory agencies will be crucial in addressing ongoing challenges and ensuring that the needs of the HAE community remain a priority on the global health agenda.
Market Trends and Forecast: Growth in HAE Therapeutics (Estimated 8-12% CAGR through 2030)
The market for Hereditary Angioedema (HAE) therapeutics is poised for robust growth through 2030, with industry analysts and stakeholders projecting a compound annual growth rate (CAGR) in the range of 8-12%. This expansion is driven by several converging factors, including increased disease awareness, improved diagnostic capabilities, and the introduction of novel therapies that address both acute attacks and long-term prophylaxis.
HAE is a rare genetic disorder characterized by recurrent episodes of severe swelling, most commonly affecting the limbs, face, intestinal tract, and airway. Historically, limited treatment options and underdiagnosis hampered patient outcomes. However, the past decade has seen a transformation in the therapeutic landscape, with the approval of targeted therapies such as C1 esterase inhibitor (C1-INH) concentrates, kallikrein inhibitors, and bradykinin receptor antagonists. These advances have significantly improved quality of life and reduced morbidity for patients.
Looking ahead to 2025 and beyond, the HAE therapeutics market is expected to benefit from several key trends:
- Pipeline Innovation: Multiple pharmaceutical companies are advancing next-generation therapies, including subcutaneous and oral formulations, gene therapies, and long-acting biologics. These innovations aim to further reduce attack frequency, improve convenience, and enhance adherence.
- Geographic Expansion: While North America and Europe currently dominate the market, increased regulatory approvals and awareness campaigns are expanding access in Asia-Pacific, Latin America, and the Middle East.
- Patient Advocacy and Education: Organizations such as the US Hereditary Angioedema Association and the HAE International are playing a pivotal role in raising awareness, supporting research, and facilitating earlier diagnosis, which in turn drives demand for therapeutics.
- Regulatory Support: Agencies like the U.S. Food and Drug Administration and the European Medicines Agency have granted orphan drug status and expedited review pathways for HAE treatments, encouraging continued investment and innovation.
Despite the positive outlook, challenges remain, including high treatment costs and the need for broader access in low- and middle-income countries. Nevertheless, with ongoing clinical trials and a strong focus on patient-centric care, the HAE therapeutics market is expected to maintain its growth trajectory, offering new hope for patients worldwide through 2030 and beyond.
Future Outlook: Innovations, Unmet Needs, and Public Health Implications
Hereditary angioedema (HAE) is a rare, potentially life-threatening genetic disorder characterized by recurrent episodes of severe swelling in various body parts. As of 2025, the landscape for HAE is rapidly evolving, with significant innovations, persistent unmet needs, and important public health considerations shaping the future outlook.
Recent years have seen the approval and clinical integration of several targeted therapies, including plasma-derived and recombinant C1-inhibitor concentrates, kallikrein inhibitors, and bradykinin receptor antagonists. These advances have transformed acute attack management and prophylaxis, reducing morbidity and mortality. Notably, the development of subcutaneous and oral therapies has improved patient convenience and adherence, with ongoing trials exploring next-generation molecules and gene therapies. For example, gene-editing approaches such as CRISPR/Cas9 are under investigation, aiming to provide a potential cure by correcting the underlying genetic defect responsible for HAE. Early-phase clinical data are anticipated within the next few years, which could mark a paradigm shift in disease management.
Despite these advances, several unmet needs persist. Many patients still experience diagnostic delays, sometimes exceeding a decade, due to the rarity and variable presentation of HAE. This delay can result in unnecessary procedures and increased risk of fatal laryngeal attacks. There is also a need for therapies suitable for pediatric populations, pregnant women, and individuals with comorbidities, as well as for treatments that are globally accessible and affordable. The high cost of current therapies remains a significant barrier, particularly in low- and middle-income countries, where access to specialized care and medications is limited.
From a public health perspective, increasing awareness among healthcare professionals and the public is crucial to improving early diagnosis and management. International organizations such as the World Health Organization and patient advocacy groups like the US Hereditary Angioedema Association are working to disseminate educational resources and support research initiatives. The establishment of global patient registries and collaborative research networks is expected to enhance understanding of disease epidemiology, natural history, and treatment outcomes, informing future guidelines and policy decisions.
Looking ahead, the next few years are likely to bring further innovation in HAE therapeutics, including the potential for one-time curative treatments. However, addressing disparities in diagnosis, access, and affordability will be essential to ensure that these advances translate into improved outcomes for all individuals living with HAE worldwide.
Sources & References
- Hereditary Angioedema International
- American College of Allergy, Asthma & Immunology
- National Center for Biotechnology Information
- US Hereditary Angioedema Association
- World Health Organization
- World Allergy Organization
- American Academy of Allergy, Asthma & Immunology
- Takeda
- CSL
- European Medicines Agency
- US Hereditary Angioedema Association
- HAE UK
- European Academy of Allergy and Clinical Immunology
- European Medicines Agency