Intravascular Visualization Systems in 2025: Transforming Cardiovascular Care with Advanced Imaging. Explore Market Growth, Disruptive Technologies, and Strategic Forecasts for the Next Five Years.
- Executive Summary: Key Findings & Market Highlights
- Market Overview: Definition, Scope, and Segmentation
- 2025 Market Size & Growth Forecast (2025–2030): 12% CAGR Analysis
- Drivers & Challenges: Factors Shaping the Intravascular Visualization Sector
- Technological Innovations: AI, 3D Imaging, and Real-Time Visualization
- Competitive Landscape: Leading Players & Emerging Entrants
- Regulatory Environment & Reimbursement Trends
- Regional Analysis: North America, Europe, Asia-Pacific, and Rest of World
- End-User Insights: Hospitals, Specialty Clinics, and Research Centers
- Future Outlook: Disruptive Trends and Strategic Opportunities to 2030
- Appendix: Methodology, Data Sources, and Glossary
- Sources & References
Executive Summary: Key Findings & Market Highlights
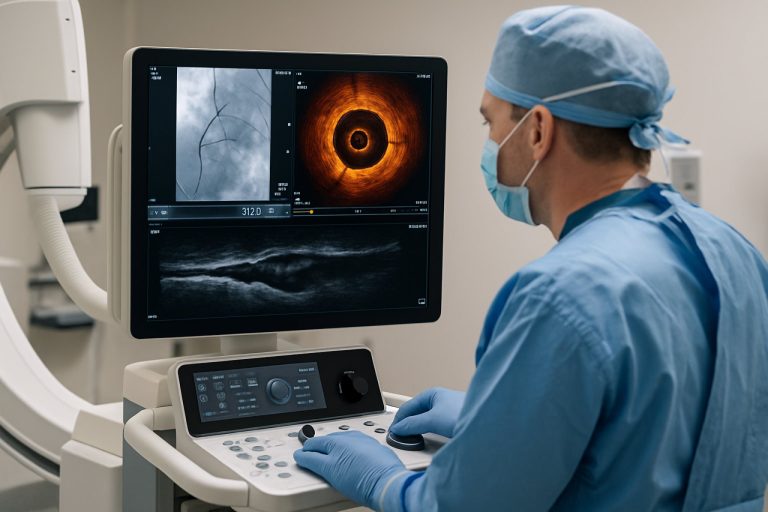
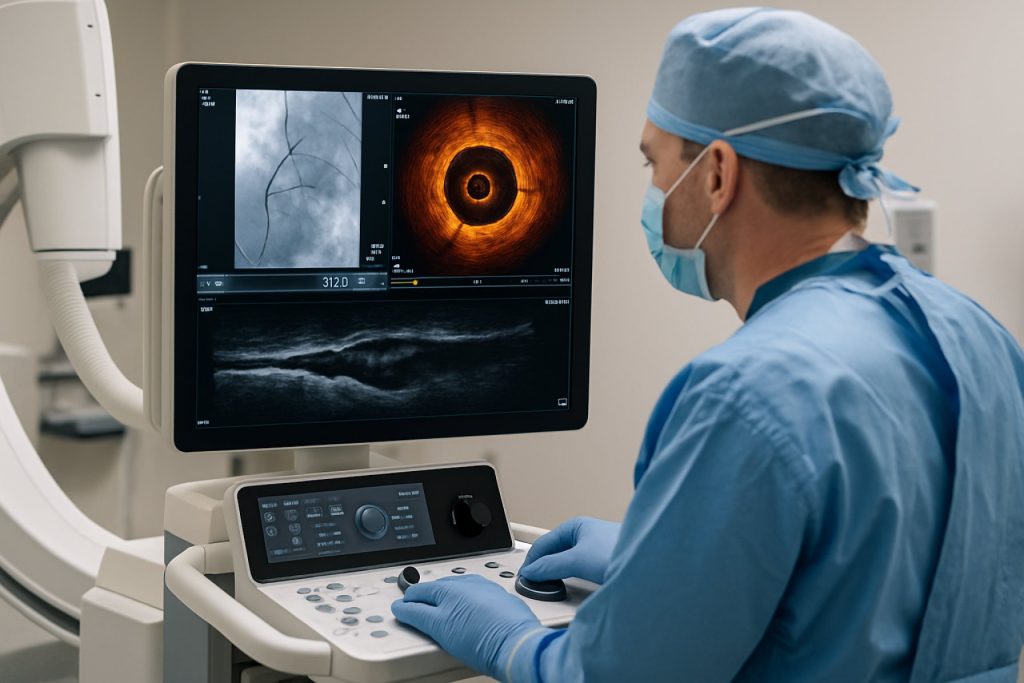
The global market for intravascular visualization systems is poised for significant growth in 2025, driven by technological advancements, rising prevalence of cardiovascular diseases, and increasing adoption of minimally invasive procedures. Intravascular visualization systems, which include intravascular ultrasound (IVUS), optical coherence tomography (OCT), and near-infrared spectroscopy (NIRS), are critical tools for real-time imaging and assessment of vascular conditions during diagnostic and interventional procedures.
Key findings indicate that the integration of artificial intelligence and advanced imaging software is enhancing the accuracy and efficiency of these systems, leading to improved clinical outcomes. Major industry players such as Philips, Boston Scientific Corporation, and Abbott are investing heavily in research and development to introduce next-generation devices with enhanced image resolution and user-friendly interfaces.
Market highlights for 2025 include a surge in demand for intravascular visualization systems in emerging economies, attributed to expanding healthcare infrastructure and increased awareness of advanced cardiovascular diagnostics. Hospitals and specialty clinics remain the primary end-users, with a notable uptick in ambulatory surgical centers adopting these technologies for outpatient procedures. Furthermore, regulatory approvals and favorable reimbursement policies in key markets such as the United States, Europe, and parts of Asia-Pacific are accelerating product adoption.
Challenges persist, including the high cost of advanced imaging systems and the need for specialized training for healthcare professionals. However, ongoing collaborations between device manufacturers and healthcare providers are addressing these barriers through educational initiatives and cost-effective product offerings.
In summary, the intravascular visualization systems market in 2025 is characterized by robust innovation, expanding clinical applications, and a growing emphasis on precision medicine. The competitive landscape is marked by strategic partnerships, product launches, and geographic expansion, positioning the sector for continued growth and improved patient care outcomes.
Market Overview: Definition, Scope, and Segmentation
Intravascular visualization systems are advanced medical imaging technologies designed to provide real-time, high-resolution images of the interior of blood vessels. These systems play a crucial role in the diagnosis, assessment, and treatment of various cardiovascular diseases by enabling clinicians to visualize vessel morphology, plaque composition, and stent placement with greater accuracy than traditional imaging modalities. The market for intravascular visualization systems is expanding rapidly, driven by the rising prevalence of cardiovascular diseases, technological advancements, and the growing adoption of minimally invasive procedures.
The scope of the intravascular visualization systems market encompasses a range of imaging modalities, including intravascular ultrasound (IVUS), optical coherence tomography (OCT), and near-infrared spectroscopy (NIRS). These technologies are primarily used in interventional cardiology and radiology for procedures such as percutaneous coronary intervention (PCI), peripheral artery disease (PAD) management, and structural heart interventions. The market also includes associated hardware, software, and disposable components required for image acquisition and analysis.
Segmentation of the intravascular visualization systems market is typically based on technology, application, end user, and geography. By technology, the market is divided into IVUS, OCT, and other emerging modalities. Application-wise, the systems are used for coronary artery disease, peripheral vascular disease, and other vascular conditions. End users include hospitals, ambulatory surgical centers, and specialty clinics. Geographically, the market is segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa, with North America currently holding a significant share due to advanced healthcare infrastructure and high adoption rates of innovative medical devices.
Key players in the market, such as Koninklijke Philips N.V., Boston Scientific Corporation, and Abbott Laboratories, are investing in research and development to enhance imaging resolution, reduce procedure times, and improve patient outcomes. The integration of artificial intelligence and machine learning into imaging platforms is also expected to drive future growth by enabling automated image interpretation and decision support.
Overall, the intravascular visualization systems market in 2025 is characterized by technological innovation, expanding clinical applications, and increasing demand for precision-guided interventions, positioning it as a vital segment within the broader cardiovascular imaging landscape.
2025 Market Size & Growth Forecast (2025–2030): 12% CAGR Analysis
The global market for intravascular visualization systems is poised for robust expansion in 2025, with projections indicating a compound annual growth rate (CAGR) of approximately 12% from 2025 to 2030. This growth trajectory is driven by the increasing adoption of advanced imaging modalities such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) in interventional cardiology and peripheral vascular procedures. These technologies enable clinicians to obtain high-resolution, real-time images of vessel walls and lumens, facilitating precise diagnosis and optimized treatment strategies.
Key market players, including Philips, Boston Scientific Corporation, and Terumo Corporation, are investing heavily in research and development to enhance the accuracy, usability, and integration of their intravascular visualization platforms. The introduction of next-generation systems with improved image clarity, user-friendly interfaces, and compatibility with digital health records is expected to accelerate market penetration, particularly in developed healthcare markets.
The rising prevalence of cardiovascular diseases, coupled with the global trend toward minimally invasive procedures, is a significant growth catalyst. Hospitals and specialty clinics are increasingly incorporating intravascular visualization systems to improve patient outcomes and reduce procedural complications. Additionally, favorable reimbursement policies in regions such as North America and Europe are supporting wider adoption.
Emerging markets in Asia-Pacific and Latin America are anticipated to contribute substantially to overall market growth, driven by expanding healthcare infrastructure and increasing awareness of advanced cardiovascular imaging technologies. Strategic collaborations between device manufacturers and healthcare providers are further facilitating technology transfer and training initiatives in these regions.
By 2030, the intravascular visualization systems market is expected to reach new heights, with technological advancements such as artificial intelligence integration and real-time 3D imaging likely to redefine clinical workflows. The sustained 12% CAGR reflects both the growing clinical demand and the ongoing innovation within the sector, positioning intravascular visualization systems as a cornerstone of modern vascular intervention.
Drivers & Challenges: Factors Shaping the Intravascular Visualization Sector
The intravascular visualization systems sector is shaped by a dynamic interplay of drivers and challenges that influence its growth trajectory and technological evolution. One of the primary drivers is the rising prevalence of cardiovascular diseases globally, which has intensified the demand for advanced diagnostic and interventional tools. The increasing adoption of minimally invasive procedures, supported by the superior imaging capabilities of intravascular ultrasound (IVUS) and optical coherence tomography (OCT), is further propelling market expansion. These technologies enable clinicians to obtain high-resolution, real-time images of vessel walls, facilitating precise assessment and treatment planning, which is particularly valuable in complex cases such as coronary artery disease and peripheral vascular interventions.
Technological advancements are another significant driver. Continuous innovation in catheter design, imaging software, and integration with other modalities has enhanced the accuracy, usability, and safety of intravascular visualization systems. For instance, the development of hybrid imaging platforms and artificial intelligence-powered analysis tools is streamlining workflow and improving diagnostic confidence. Leading manufacturers such as Philips and Boston Scientific Corporation are investing heavily in research and development to introduce next-generation systems that offer improved image quality and user experience.
However, the sector faces notable challenges. High capital and operational costs associated with intravascular visualization systems can limit adoption, particularly in resource-constrained healthcare settings. The need for specialized training and expertise to operate these sophisticated devices also poses a barrier, as does the variability in reimbursement policies across different regions. Regulatory hurdles and the lengthy approval process for new devices can further delay market entry and innovation.
Despite these challenges, ongoing collaborations between industry leaders, healthcare providers, and regulatory bodies are fostering an environment conducive to growth. Initiatives by organizations such as the U.S. Food and Drug Administration (FDA) to streamline device approvals and promote safety standards are expected to support market expansion. As the sector continues to evolve, addressing cost and training barriers while leveraging technological advancements will be crucial for the widespread adoption and clinical integration of intravascular visualization systems.
Technological Innovations: AI, 3D Imaging, and Real-Time Visualization
Technological advancements are rapidly transforming intravascular visualization systems, with artificial intelligence (AI), 3D imaging, and real-time visualization at the forefront of innovation in 2025. These technologies are enhancing the precision, efficiency, and safety of cardiovascular interventions, offering clinicians unprecedented insights into vascular anatomy and pathology.
AI-driven algorithms are increasingly integrated into intravascular imaging platforms, enabling automated detection and characterization of lesions, plaque composition, and vessel morphology. For example, Philips has incorporated AI into its intravascular ultrasound (IVUS) and optical coherence tomography (OCT) systems, streamlining image interpretation and supporting clinical decision-making. These AI tools can rapidly analyze large datasets, reduce operator variability, and provide real-time feedback during procedures.
3D imaging is another transformative innovation, allowing clinicians to visualize complex vascular structures in three dimensions. By reconstructing cross-sectional images from IVUS or OCT data, systems from companies like Boston Scientific Corporation enable detailed assessment of vessel geometry, stent placement, and lesion morphology. This enhanced visualization supports more accurate device sizing and placement, reducing the risk of complications and improving patient outcomes.
Real-time visualization technologies are also advancing, with high-resolution displays and rapid image processing now standard in leading systems. Abbott has developed platforms that provide instantaneous feedback during percutaneous coronary interventions, allowing clinicians to adjust their approach dynamically. These systems often integrate with cath lab workflow software, facilitating seamless data sharing and procedural documentation.
The convergence of AI, 3D imaging, and real-time visualization is fostering a new era of precision medicine in interventional cardiology. As these technologies continue to evolve, they are expected to further reduce procedure times, enhance diagnostic accuracy, and improve long-term patient outcomes. Ongoing collaboration between device manufacturers, clinicians, and regulatory bodies will be crucial to ensure the safe and effective integration of these innovations into routine clinical practice.
Competitive Landscape: Leading Players & Emerging Entrants
The competitive landscape of intravascular visualization systems in 2025 is characterized by the dominance of established medical device manufacturers alongside a dynamic influx of innovative startups. Major players such as Boston Scientific Corporation, Koninklijke Philips N.V., and Abbott Laboratories continue to lead the market, leveraging their extensive portfolios, global distribution networks, and ongoing investments in research and development. These companies offer a range of intravascular imaging modalities, including intravascular ultrasound (IVUS) and optical coherence tomography (OCT), which are widely adopted in interventional cardiology for real-time vessel assessment and guidance.
In recent years, Terumo Corporation and Siemens Healthineers AG have expanded their presence, focusing on technological advancements such as high-resolution imaging, integration with artificial intelligence, and improved workflow compatibility with existing catheterization lab infrastructure. These enhancements aim to provide clinicians with more precise diagnostic information and facilitate minimally invasive procedures.
Emerging entrants are increasingly shaping the competitive dynamics by introducing novel imaging platforms and software-driven solutions. Startups and smaller firms are focusing on artificial intelligence-powered image analysis, miniaturized catheter designs, and hybrid imaging systems that combine multiple modalities for comprehensive vessel visualization. Collaborations between these innovators and established industry leaders are common, as larger companies seek to incorporate disruptive technologies into their product lines through partnerships or acquisitions.
The competitive environment is further influenced by regulatory approvals and reimbursement policies, which can accelerate or hinder market entry for new devices. Companies with robust clinical evidence supporting the efficacy and safety of their systems are better positioned to gain market share, particularly in regions with stringent regulatory requirements such as the United States and the European Union.
Overall, the intravascular visualization systems market in 2025 is marked by intense competition, rapid technological evolution, and a growing emphasis on integrated, data-driven solutions. The interplay between established leaders and agile newcomers is expected to drive continued innovation, ultimately enhancing patient outcomes in cardiovascular interventions.
Regulatory Environment & Reimbursement Trends
The regulatory environment for intravascular visualization systems—such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT)—continues to evolve in 2025, reflecting advances in technology and the growing demand for precision in cardiovascular interventions. Regulatory agencies, including the U.S. Food and Drug Administration (FDA) and the European Commission, have established rigorous pathways for the approval and post-market surveillance of these devices, emphasizing safety, efficacy, and clinical benefit.
In the United States, the FDA classifies most intravascular visualization systems as Class II or III medical devices, requiring premarket notification (510(k)) or premarket approval (PMA) depending on the device’s risk profile and novelty. Recent years have seen a trend toward more streamlined review processes for devices that demonstrate substantial equivalence to predicate technologies, particularly when supported by robust clinical data. The FDA also encourages the use of real-world evidence and post-market registries to monitor device performance and inform future regulatory decisions.
In Europe, the implementation of the Medical Device Regulation (MDR) has introduced stricter requirements for clinical evaluation, technical documentation, and post-market surveillance. Manufacturers must now provide comprehensive evidence of safety and performance, including data from clinical investigations and post-market clinical follow-up. Notified Bodies play a central role in the conformity assessment process, and the European Medicines Agency (EMA) collaborates on combination products involving drugs and devices.
Reimbursement trends are also shifting, with payers increasingly demanding evidence of cost-effectiveness and improved patient outcomes. In the U.S., the Centers for Medicare & Medicaid Services (CMS) has established specific reimbursement codes for IVUS and OCT procedures, but coverage decisions may vary by region and payer. Value-based care models are incentivizing the adoption of technologies that reduce complications, shorten hospital stays, and lower overall healthcare costs. In Europe, national health systems and private insurers are similarly scrutinizing the clinical and economic value of intravascular visualization systems before granting reimbursement.
Overall, the regulatory and reimbursement landscape in 2025 is characterized by heightened scrutiny, a focus on real-world evidence, and a growing emphasis on demonstrating both clinical and economic value. Manufacturers must navigate these evolving requirements to ensure successful market access and adoption of their intravascular visualization technologies.
Regional Analysis: North America, Europe, Asia-Pacific, and Rest of World
The global market for intravascular visualization systems is characterized by significant regional variations, driven by differences in healthcare infrastructure, regulatory environments, and adoption rates of advanced medical technologies. In 2025, North America continues to lead the market, propelled by robust investments in healthcare, a high prevalence of cardiovascular diseases, and early adoption of innovative imaging modalities. The presence of major industry players such as Boston Scientific Corporation and Koninklijke Philips N.V. further strengthens the region’s dominance, as these companies drive research, development, and commercialization of next-generation intravascular imaging solutions.
Europe follows closely, benefiting from well-established healthcare systems and supportive regulatory frameworks that facilitate the integration of intravascular visualization technologies into routine clinical practice. Countries like Germany, the United Kingdom, and France are at the forefront, with increasing adoption of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) for complex coronary interventions. The European market is also shaped by collaborations between hospitals and technology providers, such as Siemens Healthineers AG, to enhance procedural outcomes and patient safety.
The Asia-Pacific region is experiencing the fastest growth, fueled by rising healthcare expenditures, expanding access to advanced medical devices, and a growing burden of cardiovascular diseases. Markets such as China, Japan, and India are witnessing increased investments in hospital infrastructure and training, which are critical for the widespread adoption of intravascular visualization systems. Local manufacturers and subsidiaries of global companies, including Terumo Corporation, are playing a pivotal role in making these technologies more accessible and affordable across the region.
The Rest of the World, encompassing Latin America, the Middle East, and Africa, presents a more heterogeneous landscape. While adoption rates remain comparatively lower due to budgetary constraints and limited specialist expertise, there is a gradual uptick in demand, particularly in urban centers and private healthcare facilities. International partnerships and government-led initiatives aimed at improving cardiovascular care are expected to drive incremental growth in these emerging markets.
Overall, regional dynamics in 2025 reflect a combination of technological innovation, healthcare policy, and economic factors, with North America and Europe maintaining leadership, Asia-Pacific rapidly expanding, and the Rest of the World showing promising, albeit slower, progress in the adoption of intravascular visualization systems.
End-User Insights: Hospitals, Specialty Clinics, and Research Centers
End-users such as hospitals, specialty clinics, and research centers play a pivotal role in the adoption and evolution of intravascular visualization systems. These advanced imaging technologies, including intravascular ultrasound (IVUS) and optical coherence tomography (OCT), are increasingly integrated into clinical workflows to enhance the diagnosis and treatment of cardiovascular diseases.
Hospitals represent the largest segment of end-users, driven by the high volume of interventional cardiology procedures and the need for precise imaging to guide stent placement, assess lesion morphology, and minimize procedural risks. Leading healthcare institutions, such as those affiliated with the American Hospital Association, are investing in state-of-the-art intravascular visualization systems to improve patient outcomes and support evidence-based practices. The integration of these systems with electronic health records and catheterization lab equipment further streamlines workflow and data management.
Specialty clinics, particularly those focused on cardiology and vascular interventions, are also significant adopters. These clinics benefit from the portability and user-friendly interfaces of modern intravascular imaging devices, which allow for rapid diagnostics and minimally invasive procedures. The ability to offer advanced imaging services enhances the clinic’s reputation and attracts patients seeking specialized care. Organizations such as the American College of Cardiology provide guidelines and training to ensure optimal use of these technologies in outpatient settings.
Research centers contribute to the advancement of intravascular visualization systems by conducting clinical trials, validating new imaging modalities, and exploring novel applications. Collaboration between academic institutions and industry leaders, such as Philips and Boston Scientific Corporation, accelerates innovation and supports the development of next-generation devices. Research centers also play a key role in generating real-world evidence and publishing findings that inform clinical guidelines and regulatory approvals.
Overall, the insights and feedback from these end-user groups are instrumental in shaping product development, regulatory standards, and best practices for intravascular visualization systems. Their collective experience ensures that these technologies continue to evolve in alignment with clinical needs and patient safety requirements.
Future Outlook: Disruptive Trends and Strategic Opportunities to 2030
The future of intravascular visualization systems is poised for significant transformation through 2030, driven by rapid technological advancements, evolving clinical needs, and strategic industry shifts. As cardiovascular diseases remain a leading cause of morbidity and mortality worldwide, the demand for precise, real-time imaging within blood vessels is intensifying. This is catalyzing innovation in modalities such as intravascular ultrasound (IVUS), optical coherence tomography (OCT), and emerging hybrid systems.
One of the most disruptive trends is the integration of artificial intelligence (AI) and machine learning algorithms into visualization platforms. These technologies are expected to enhance image interpretation, automate lesion assessment, and support clinical decision-making, thereby improving procedural outcomes and reducing operator variability. Companies like Philips and Boston Scientific Corporation are actively investing in AI-driven solutions to augment their intravascular imaging portfolios.
Miniaturization and improved catheter design are also shaping the future landscape. Next-generation devices are becoming more flexible, with higher resolution and faster data acquisition, enabling safer navigation through complex anatomies and more detailed visualization of vessel walls. The development of wireless and disposable imaging catheters is anticipated to streamline workflows and reduce infection risks, aligning with broader trends in minimally invasive interventions.
Hybrid imaging systems that combine IVUS, OCT, and even near-infrared spectroscopy (NIRS) are emerging as powerful tools for comprehensive plaque characterization and risk stratification. These multimodal platforms are expected to facilitate personalized treatment strategies and support the growing emphasis on precision medicine in interventional cardiology.
Strategically, partnerships between device manufacturers, software developers, and healthcare providers are accelerating the adoption of advanced visualization systems. Initiatives by organizations such as the American College of Cardiology to standardize imaging protocols and promote evidence-based use are likely to further drive market growth and clinical integration.
Looking ahead to 2030, the convergence of AI, miniaturization, and hybrid imaging is set to redefine intravascular visualization. Companies that prioritize interoperability, data security, and clinician training will be best positioned to capitalize on these opportunities, ultimately improving patient outcomes and shaping the future of cardiovascular care.
Appendix: Methodology, Data Sources, and Glossary
This appendix outlines the methodology, data sources, and glossary relevant to the analysis of intravascular visualization systems for 2025.
- Methodology: The research employed a mixed-methods approach, combining quantitative data from regulatory filings, product registries, and sales reports with qualitative insights from clinical guidelines and expert interviews. Market sizing and trend analysis were based on publicly available financial statements, product launch announcements, and procurement data from leading manufacturers and healthcare providers. The study prioritized peer-reviewed clinical studies and official guidance from regulatory agencies to assess the clinical efficacy and adoption of intravascular visualization technologies.
- Data Sources: Primary data sources included annual and quarterly reports from major manufacturers such as Boston Scientific Corporation, Koninklijke Philips N.V., and Terumo Corporation. Regulatory and clinical data were obtained from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Additional information was sourced from professional societies, including the American College of Cardiology (ACC) and the European Society of Cardiology (ESC). Where possible, data were cross-verified with procurement records from major hospital networks and purchasing groups.
-
Glossary:
- Intravascular Visualization Systems: Medical imaging devices used to visualize the interior of blood vessels, including intravascular ultrasound (IVUS) and optical coherence tomography (OCT) systems.
- IVUS (Intravascular Ultrasound): A catheter-based imaging technology that uses ultrasound to provide cross-sectional images of blood vessels.
- OCT (Optical Coherence Tomography): An imaging modality that uses light waves to capture high-resolution images of vessel walls.
- Regulatory Approval: Official authorization from agencies such as the FDA or EMA for the clinical use of medical devices.
- Clinical Guidelines: Evidence-based recommendations for the use of medical technologies in patient care, issued by professional societies.
Sources & References
- Philips
- Boston Scientific Corporation
- Terumo Corporation
- Siemens Healthineers AG
- European Commission
- European Medicines Agency (EMA)
- Centers for Medicare & Medicaid Services (CMS)
- American Hospital Association
- American College of Cardiology